To prevent insect-borne diseases, and address the threat of insecticide resistance, novel and improved public health insecticides, formulations and products are needed.
A Vector Expedited Review Voucher (VERV) creates a new incentive for manufacturers who currently do not discover and develop novel new insecticides because the market for public health insecticides does not provide an adequate return on the development costs.
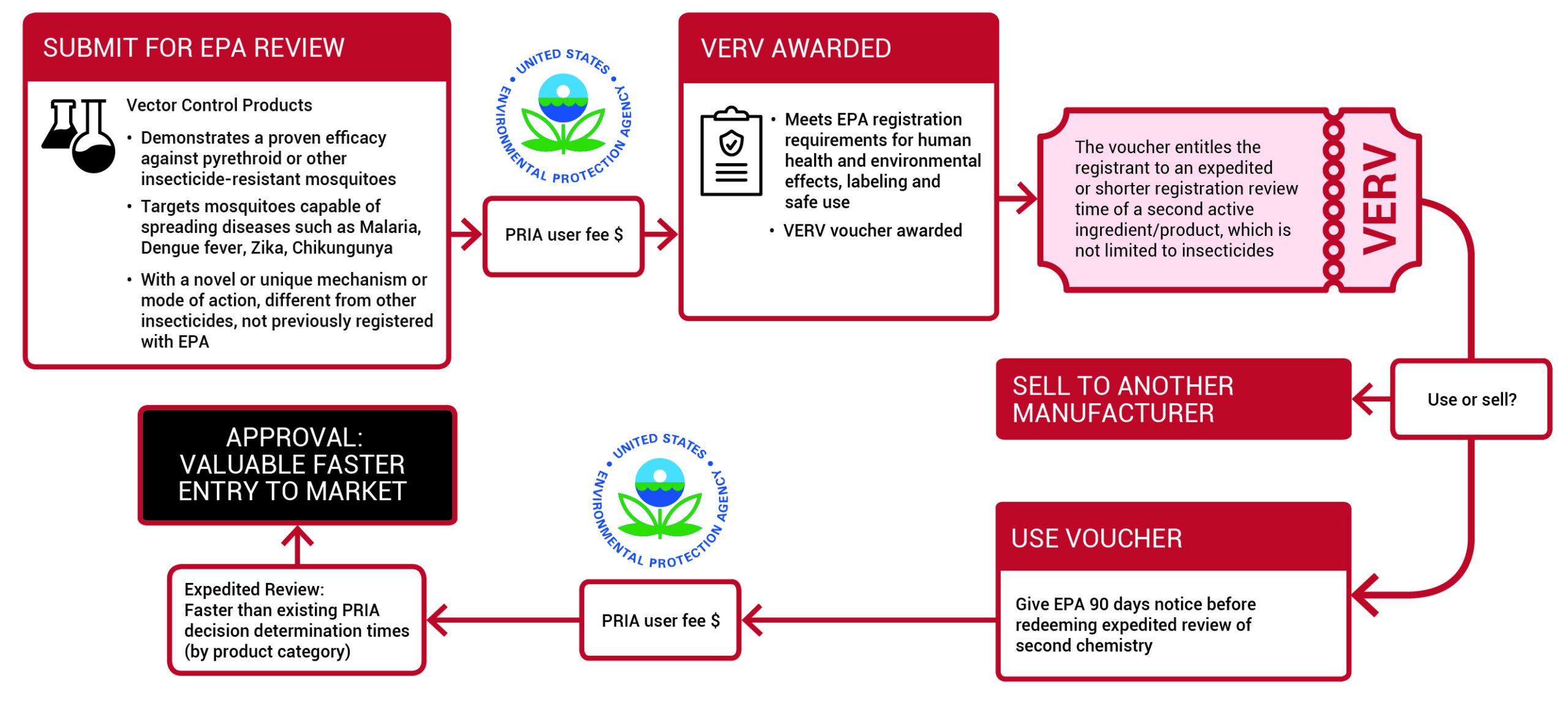
How will it work?
VERV rewards the manufacturer of a new and novel public-use insecticide with a voucher to receive an expedited U.S. Environmental Protection Agency (EPA) registration review of a second, more profitable product with no sacrifices in safety or thoroughness. Getting this second chemistry to market faster allows the registrant the opportunity to generate a financial return to mitigate the development costs and potential losses on the first chemistry. The expedited review is valuable because it increases the speed to market of the second chemistry and acts as an incentive to invest in novel new insecticides for insect-borne diseases.
The development of a totally novel insecticide from discovery through to launch can cost between $100-$250Million and take more than twelve years, making a return on investment in vector control markets almost impossible. Awarding a VERV gives an innovator company an opportunity to generate a financial return on another product as well as reducing the time to market of critically important public health insecticides.
VERV is modelled after the Priority Review Voucher (PRV) programme (Sec. 524 2007 FDA Amendments Act) that is a proven incentive for neglected tropical diseases administered by the Food and Drug Administration (FDA) since 2008. The programme has no cost to taxpayers and the FDA recoups its review costs with a special user fee. PRV’s have been exercised by winning companies to bring a new drug more quickly to US patients and vouchers have also been sold to other manufacturers for hundreds of millions of dollars. This value to manufacturers is a needed incentive to promote discovery and development of much needed treatments for malaria and neglected tropical diseases.